Strong combination, the general-purpose CAR-T is expected to treat a variety of cancers
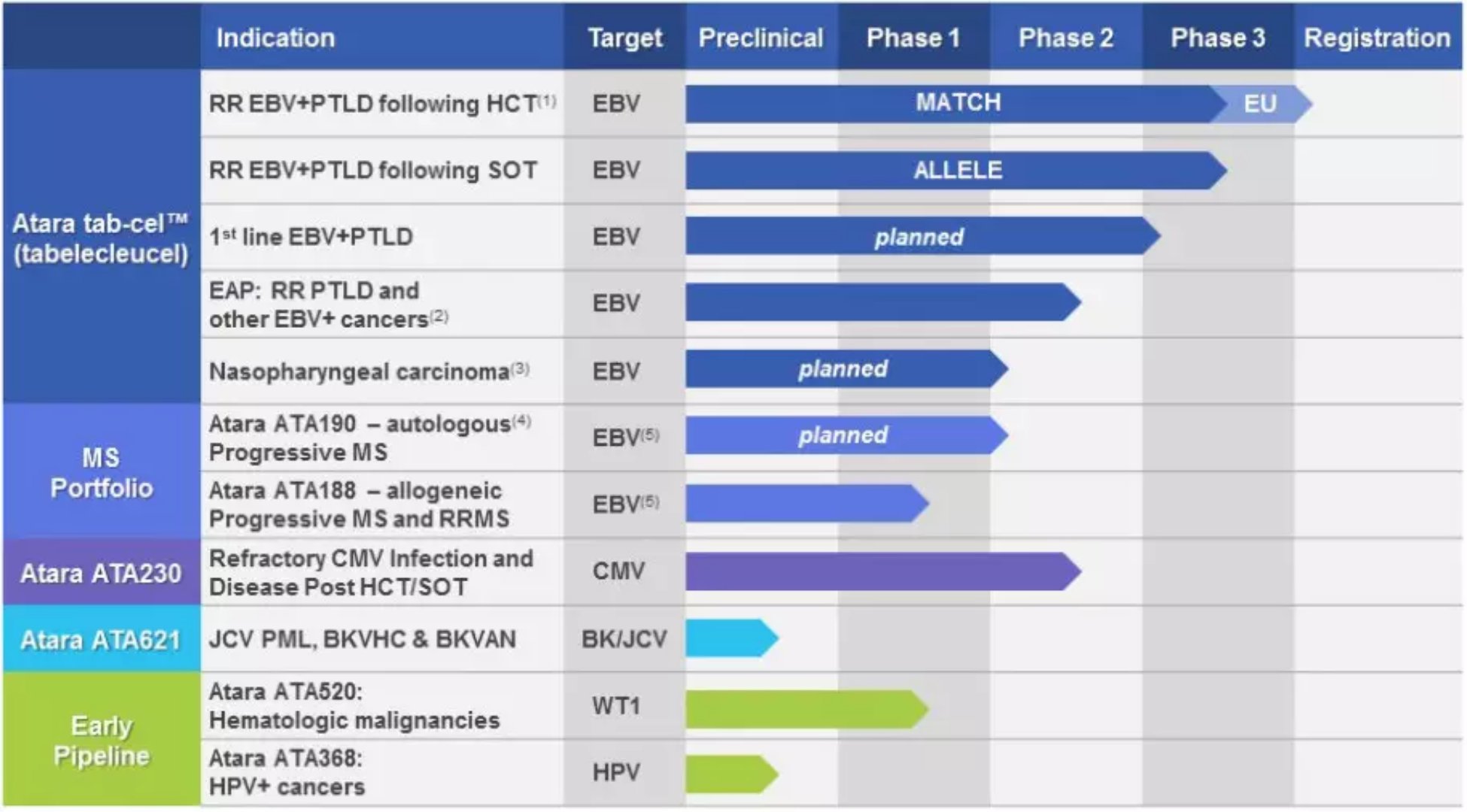
Strong combination, the general-purpose CAR-T is expected to treat a variety of cancers May 09, 2018 Source: WuXi PharmaTech Atra Biotherapeutics, a T-cell immunotherapy company, today announced the expansion of its collaboration with the Memorial Sloan Kettering (MSK) Cancer Center to develop the next generation of genetically engineered chimeric antigen receptor T cell (CAR-T) immunotherapy. The agreement is Atara's next strategy, using the company's technology platform to develop off-the-shelf allogeneic T-cell immunotherapy to change the lives of patients with severe illness. Atara Biotherapeutics' universal allogeneic T cells are provided and bioengineered by volunteers with healthy immune function. Fast delivery from stock to patient without pre-treatment is possible. Atara's T-cell immunotherapy aims to accurately identify and eliminate cancer or diseased cells without affecting healthy cells. Atara is developing an advanced T cell immunotherapy tabelecleucel (tab-cel, ATA129) for the treatment of Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disorders (EBV + PTLD) and including nasopharyngeal carcinoma (NPC) Other EBV-related blood and solid tumors were ineffective after receiving rituximab (rituximab). Atara is also developing universal allogeneic ATA188 and autologous ATA190 T cell immunotherapy to treat multiple sclerosis (MS) against specific EBV antigens using complementary targeted antigen recognition technology. Atara also initiated a multinational Phase 1 ATA188 clinical study in Australia and the United States for patients with progressive or relapsing-remitting MS. Its clinical pipeline also includes ATA520 for Wilms Tumor 1 (WT1) and ATA230 for cytomegalovirus (CMV). Under the agreement, Atara will receive several innovative technologies from MSK, including a new CAR-T construct with physiological T cell activation properties and a method for designing CAR-T immunotherapy. Atara also signed an exclusive research collaboration with Dr. Michel Sadelain, Director of the Cell Engineering Center at MSK, to develop a new CAR-T immunotherapy using next-generation technology for oncology and autoimmune diseases. And infectious diseases. â–² Atara Biotherapeutics' Multiple T Cell Immunotherapy Candidates (Source: Atara Biotherapeutics Official Website) Dr. Michel Sadelain said: "We are very eager to work with Atara to continue to advance MSK's promising allogeneic T cell immunotherapy technology. The new CAR-T technology seeks to overcome ongoing therapeutic challenges such as safety, tolerability, and therapeutic response. Persistence, and the major medical needs that are not met by the first generation of CAR-T immunotherapy." Dr. Isaac Ciechanover, President and CEO of Atara, said: "Our previous collaboration with MSK was very efficient, especially at Atara's general-purpose allogeneic T-cell immunotherapy tab-cel in Phase 3 clinical development. Our deep collaboration with MSK We are able to use the technology platform, manufacturing expertise and research and development capabilities of universal T cell immunotherapy to rapidly advance the development of new CAR-T projects for gene editing. Looking ahead, we plan to continue to develop complementary genetic engineering technologies to expand our Pipeline and realize the full potential of our platform." We hope that this cooperation will bring universal T-cell therapy to patients at an early date and is widely used in many difficult diseases. Reference materials: [1] Atara Biotherapeutics Expands T-cell Immunotherapy Collaboration to Advance Next-generation Car T Technologies in Oncology, Autoimmune and Other Diseases [2] Atara Biotherapeutics official website Automatic Massage Foot Bath Machine
The automatic massage foot bath machine is a device that uses water and massage rollers to provide a relaxing foot massage. It usually has a basin filled with water and has built-in massage rollers that move and massage the feet. The machine may also have other functions, such as heat therapy, air bubbles, and vibration. Users place their feet in the basin and the machine provides a soothing massage that helps relieve tension and improve foot circulation. Some automatic foot massage machines also come with removable attachments for additional massage options.
Automatic Massage Foot Bath Machine,Foot Spa Massageer,Heated Foot Spa,Foot Bath Massage Basin Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootbath.com