Breaking through the bottleneck of cancer immunotherapy, the answer is so simple?
Breaking through the bottleneck of cancer immunotherapy, the answer is so simple? March 29, 2019 Source: Academic Jingwei Today, scientists at the National Institutes of Health (NIH) and the National Cancer Institute (NCI) bring a heavy study and are on the cover of Science. In the study, scientists discovered a path that broke through the bottleneck of cancer immunotherapy. And their answer seems to be very simple: potassium. In the past few years, the advent of immunotherapy has greatly changed the pattern of human treatment of cancer: with the help of immunotherapy, many advanced cancers that could not be cured can also be controlled for a long time, even "functional healing." However, tumor immunotherapy is not a panacea, but has its bottleneck. One of the most critical bottlenecks is that only 20%-30% of patients respond to immunotherapy. For most cancer patients, immunotherapy does not work. Surprisingly, doctors are still able to find many immune T cells around the patient's tumor. However, these anti-cancer guardians just stayed quietly beside the tumor, and they did not launch an attack, which made people wonder. For years, scientists have been trying to find the reasons behind this phenomenon. The current mainstream view is that the tumor microenvironment inhibits the function of immune T cells. Previously, Dr. Nicholas Restifo, a cancer immunologist, led the team to explore the mysteries of the tumor microenvironment. They found that dead cancer cells release high levels of potassium, which can cause surrounding T cells to lose their ability to attack cancer cells. In today's paper, the researchers further expanded their findings. And this discovery is expected to lead to better immunotherapy. First, the team replied that potassium is a sputum that inhibits T cell activity. They found that if the concentration of potassium ions in the environment is too high, the metabolism of T cells will be seriously affected, and it will not be able to absorb nutrients from the surrounding environment, thus being in a state of "nutrition." It is conceivable that T cells that are not even eaten by rice can naturally not effectively fight tumors. In addition, the researchers also found a very interesting phenomenon. Under the influence of potassium ions, the epigenetic modification of T cells will change, affecting the differentiation of T cells. As a result, these T cells have been tied to the "stem cell state" and will only continue to self-replicate, but cannot be successfully differentiated into killer effector T cells. This can explain that a large number of T cells that accumulate around the tumor will be indifferent to the lesion. After clarifying the biological mechanism, the words "stem cell status" attracted the attention of researchers. It turns out that people are actively developing a new type of immunotherapy. Doctors expect to isolate tumor-infiltrating immune T cells from patients, expand them in vitro, and return T cells to patients in order to initiate tumors. Fierce attack. This therapy is called "adoptive T-cell therapy" and has only been successful in a small number of patients. A number of preclinical and clinical trials have shown that the "stem cell status" of T cells is the key to the success of this therapy. Since long-term exposure to high-concentration potassium ions can maintain the "heart cells", can they bring more powerful adoptive cell therapy? To test the feasibility of this strategy, the researchers first isolated tumor-infiltrating T cells from multiple cancer patients and expanded them in an in vitro environment rich in potassium ions. Studies have confirmed their hypothesis that although potassium ions inhibit the activity of immune T cells in the tumor microenvironment, the benefits it brings to adoptive cell therapy are even more pronounced: some key biomarker levels of these T cells Significantly elevated, indicating that they may have a stronger effect. The efficacy of these cells has also been confirmed in mice. Also after treatment with potassium ions, the expanded T cells were infused back into melanoma mice. The study found that mice that did not receive any T-cell infusion died in less than 20 days; although mice that received normal T-cell infusion lived longer, the mortality rate approached 80 after more than 30 days of treatment. %. In contrast, T cells that have been subjected to potassium ion baptism in vitro exhibit a surprising therapeutic effect in the face of a poor tumor microenvironment. After more than 30 days, the survival rate of the mice was as high as 100%, and the tumor area was well controlled. Even more gratifying is that by understanding the biological pathways behind it, we are expected to use drugs directly to achieve the same effect. The researchers found that by directly hitting the key enzymes in this pathway, a molecule called 2-HC can also increase the anticancer effect of T cells. Also in the mouse model, mice that did not receive T cells died in about 20 days, and mice that received normal T cell infusion died in about 50 days. When T cells were treated with 2-HC and infused into mice for 50 days, the survival rate of these mice also reached 100%! "This study has helped us better understand the mechanisms of cancer immunotherapy and is expected to make immune therapy better and more durable," Dr. Restifo said. A review of the article in Science also pointed out that the study brought new strategies to enhance immunotherapy. In the future, these scientists are looking to launch clinical trials to test the therapeutic potential of this therapy in human patients. We look forward to hearing good news soon! 2-Piece Ostomy pouch MDK-BO-02 500ML
A one-piece colostomy bags, drainable pouching system designed to be opened at the bottom when emptying. These colostomy bag machine are most suitable for colostomies or ileostomies. Ostomy bag colostomy barrier is a standard wear skin barrier that is gentle to the skin and allows for frequent pouch removal. To close the pouch, use the curved, beige clamp. Soft, beige disposable colostomy bag pouch panels on body side help provide comfort.
The features are soft and flex,standard wear, skin barrier, flat. The Chassis made in 100% hydrocolloid No tape border. Cut-to-fit skin barrier. Ultra-clear odor-barrier pouch film. Curved, beige pouch clamp. With comfortable ware pouch panel body side only. Not made with natural rubber latex.
Ostomy urostomy colostomy bag care products consist of sodium carboxymethylcellulose, adhesives made from medical hot melt adhesives, and separator paper or separators. Nursing equipment for ileum, colon, rectum or urethral stoma,The coloplast colostomy bag comes into contact with intact skin and intestinal lumen,Non-sterile supply. Colostomy bag reusable for stoma washing, care and collection of excreta and skin care around the stoma.
Use disposable colostomy bag, make sure the skin around the stoma is clean and dry before use. Remove the bags for colostomi from the package and separator or paper. Reshape the coloplast colostomy bag into the desired shape and size to fit the skin around the stoma. Apply the colostomy convatec bag to the skin around the stoma,adjust the shape of the paste again,Gently press so that it is firmly flat on the skin. Attach the stoma colostomy bag undercarriage to the stoma. The colostomy bag care can be peeled off the skin, don`t reuse. These one-piece-colostomy bag or two-piece-colostomy-bag are non-sterile product and is valid for three years under the condition of meeting the storage conditions.
1-Piece Ostomy Pouch,One Piece Ostomy Bags,One Piece Ostomy Pouch,1 Piece Ostomy Bag Henan Maidingkang Medical Technology Co.,Ltd , https://www.mdkmedical.com
â–²This research is on the cover of Science (Source: V. Altounian/Science) 
â–² Dr. Nicholas Restifo, head of the study (Source: NCI, Bill Branson) 
â–² Potassium ions can have multiple effects on T cells (Source: Science) 
â–²This study is expected to bring better immunotherapy (Source: Science) 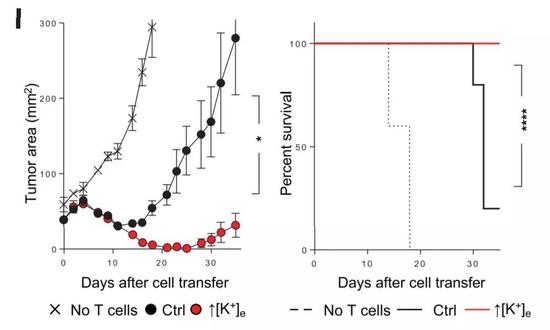
â–² After potassium ion treatment, in vitro expanded T cells show amazing anti-cancer effects in mouse models (Source: Science) 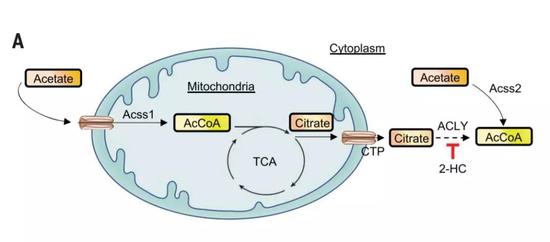
â–²The key signaling pathway of this study (Source: Science)