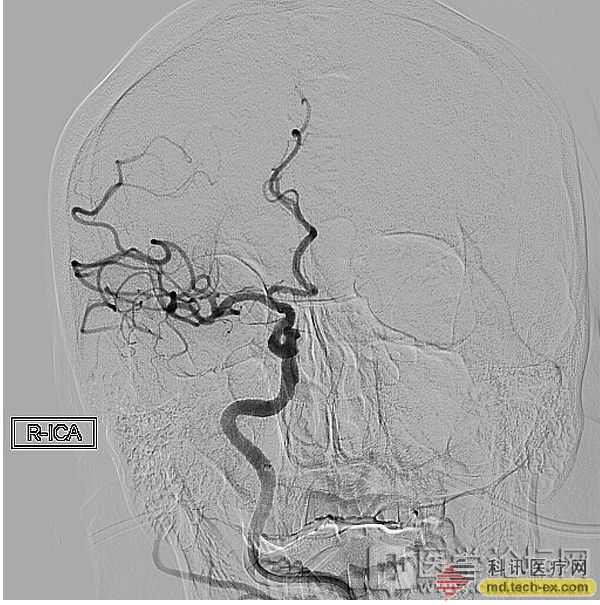
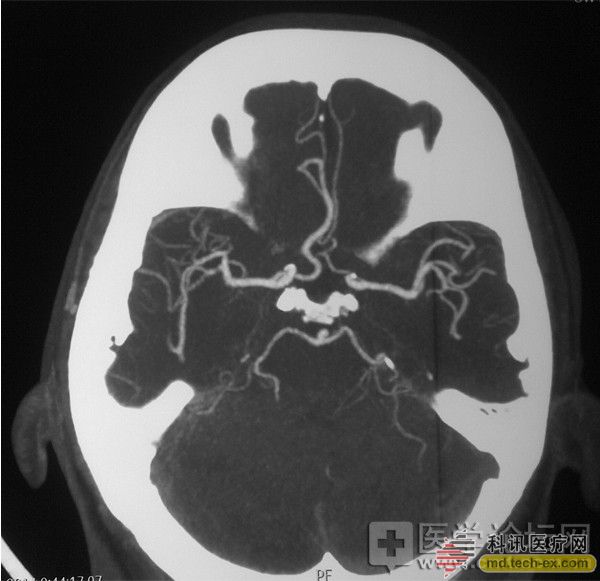
Qilu uses Ke Hui stent to remove the cerebral artery
Release date: 2014-07-02 figure 1 figure 2 image 3 Recently, an elderly male patient came to the emergency neurology department of Qilu Hospital of Shandong University because of "unclear speech and weak limbs on the left side for half an hour." The patient had previous history of alcoholic cardiomyopathy, coronary artery bypass, cardiac insufficiency, atrial fibrillation (self-deactivation of warfarin for nearly 20 days), hypertension, diabetes, and renal artery stenting. At the time of admission, the body was examined: Shen Zhiqing, gaze on the right side of the eyes, level 0 muscle strength on the left side, decreased muscle tone, and reduced limb reflex (+). The bilateral pathological signs were not elicited. The clinical diagnosis is cerebral embolism. Considering the cerebral embolism, the recanalization rate of thrombolysis is often low. After obtaining informed consent from the family, Dr. Wu Wei and Dr. Jianwei Wei, deputy directors of neurology, urgently start the acute endovascular treatment of acute cerebral infarction. Angiography revealed that the right middle cerebral artery M1 segment was completely occluded at the beginning, and the intracranial mechanical thrombectomy was performed. The intracranial vascular tortuosity was more serious and the operation was difficult. In the operation, Kehui company's special stent-type thrombectomy device ------Solitaire FR stent was used. The embolus was successfully removed 4.5 hours after the onset of the disease, and the right middle cerebral artery recovered blood flow. (Figure: 1 before treatment, after treatment in Figure 2, after treatment in Figure 3) Intra-arterial mechanical thrombectomy is a powerful weapon for re-opening of early cerebral infarction. It has the advantages of long time window, high vascular access rate and low cerebral hemorrhage rate compared with intravenous thrombolysis. The SWIFT study published in the March issue of Lancet showed that the Solitaire FR stent-type thrombectomy device compared with the first-generation thrombectomy device Merci was 61% vs 24%, respectively. Solitaire FR was approved by the US FDA for clinical use in 2012. It is the latest international cerebral artery thrombectomy device. In May 2014, it was clinically registered in our hospital. In the past, we used the aneurysm assisted stent Solitaire AB stent in the intracranial artery. It is reported that this case is the first case of intraoperative arterial thrombectomy in Solitaire FR in Shandong Province. Source: Kexun Medical Network Disposable Gel,Moisturizing Hand Sanitizer,Hand Sanitizer,Hand Sanitizer Dispenser Wuxi Keni Daily Cosmetics Co.,Ltd , https://www.kenidailycosmetics.com