Michael S. Young, Kim van Tran, Evelyn Goh and Jeremy C. Shia
Waters Corporation (Milford, MA, USA)
Application advantage
â– Rapid extraction of aminoglycoside antibiotics from dairy or meat
â– Fast LC/MS/MS analysis
â– Direct solid phase extraction (SPE) purification
â– Low ppb detection limit
Waters Solutions
ACQUITY UPLC ® System
ACQUITY ® TQD Mass Spectrometer
ACQUITY UPLC HSS PFP Column
Oasis ® HLB Extraction Column
Polypropylene sample bottle
Key words
Aminoglycoside antibiotics, solid phase extraction, dairy, beef, LC/MS/MS
introduction
Aminoglycoside antibiotics are used as veterinary drugs for the treatment of meat and milk-producing animals, and therefore require an analytical method for analyzing their residues in these commodities. Such antibiotics pose a huge challenge to residue analysis. Unlike most other antibiotics, these compounds cannot be extracted from tissues or dairy products using acetonitrile or other organic solvents. In the study, aminoglycoside antibiotics were extracted from meat tissue or dairy using an aqueous buffer containing trichloroacetic acid (TCA). The addition of TCA enables protein precipitation and inhibition of protein binding to these analytes. Prior to LC/MS analysis, an effective solid phase extraction (SPE) purification operation is used to remove residual TCA to minimize co-extraction interference. Good solid phase extraction yield and purification results are obtained in milk and meat by using Oasis HLB (Highly Efficient, Water-Immersible Reverse Phase Absorbent). In the subsequent analysis, the ACQUITY UPLC HSS PFP column was used to achieve excellent UPLC ® separation performance in reversed-phase ion pair mode. Heptafluorobutyric acid (HFBA) is used as an ion pairing reagent. The reagent is volatile and can be used in conjunction with mass spectrometry.
Globally, the maximum residue limit (MRL) of aminoglycoside antibiotics in dairy products is generally in the range of 100 to 1000 μg/kg, as shown in Figure 1. This application note provides a method for determining the level of aminoglycoside antibiotic residues in milk and bovine tissues at the ppb level, which was developed in part by the methods of References 1 and 2.
experiment
UPLC condition
System: ACQUITY UPLC
Column: ACQUITY HSS PFP 2.1 x 100 mm, 1.8 μm Part No. 186005967
Injection volume: 30 μL
Temperature: 35 ° C
Mobile phase A: 20 mM HFBA aqueous solution
Mobile phase B: 20 ​​mM HFBA acetonitrile solution
Flow rate: 0.50 mL/min
Gradient: 20% B initial conditions, linear gradient increased to 80% B in 7 minutes, held for 8 minutes, concentration dropped back to 20% B for 8.1 minutes. Rebalance and keep for 10 minutes.
Mass spectrometry condition
Mass spectrometer: ACQUITY TQD
Mode: Positive ion electrospray (ESI+)
Capillary voltage: 3.0 kV
Extraction cone voltage: 3.0 V
Ion source temperature: 130 °C
Cone gas flow rate: 20 L/h
Desolvent temperature: 450 °C
Desolvent gas flow rate: 900 L/h
Collision gas: Argon, flow rate 0.20 mL/min
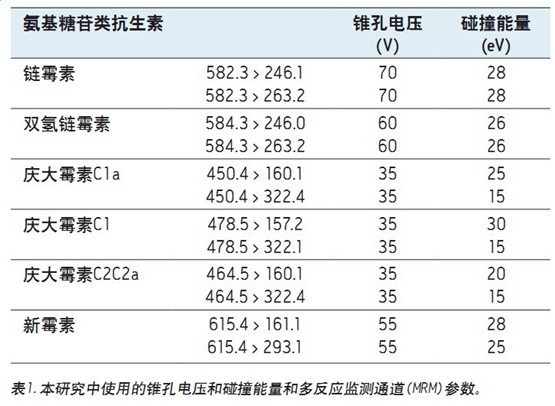
Table 1 summarizes the cone and collision parameters and multiple reaction monitoring (MRM) transitions used in this study.
Sample Preparation
Extraction Buffer (10 mM NH4OOCH3/0.4 mM Na2EDTA/1% NaCl/2% TCA):
0.77 g of ammonium acetate (NH4OOCH3) was placed in a 1 L volumetric flask. Add approximately 900 mL of reagent water and dissolve. The pH was adjusted to 4.0 using 1 N HCl or 1 N NaOH. 0.15 g of disodium edetate (Na2EDTA.2H2O), 5 g of sodium chloride (NaCl) and 20 g of trifluoroacetic acid (TCA) were added. Mix well to dissolve the solids and add reagent water to the mark.
Preliminary extraction:
Place 2 g of uniform bovine tissue or 10 mL of milk in a 50 mL centrifuge tube. Add 20 mL of extraction buffer, vortex for 10 seconds, then shake for 1 minute. The sample was centrifuged at 4000 RPM for 5 minutes and the supernatant was collected. The pH of the supernatant was adjusted to 6.5 ± 0.5 using dilute HCl or NaOH as needed.
SPE purification:
Oasis HLB 96-well plates (30 mg) were used in this study. A 1 cc/30 mg extraction column can be used if needed. Use 1.5 mL of methanol and 1.5 mL of water to equilibrate the plate well or the extraction column. Set the flow rate to 1 mL/min or lower. The pH-adjusted supernatant obtained by the preliminary extraction was added, and for the tissue sample, 1 mL of the supernatant was added, and for the dairy sample, 1.5 mL of the supernatant was added. Wash with 1 mL of water. Elute with 0.5 mL of 10:5:85 formic acid/isopropanol/water. 1.5 μL of HFBA was added and analyzed using UPLC/MS/MS.
Results and discussion
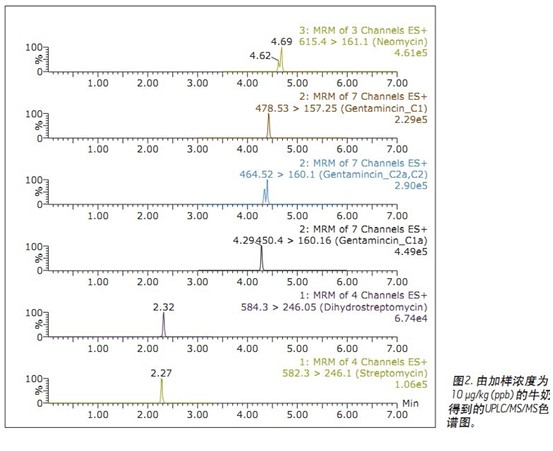
Figure 2 is a reproduced UPLC/MS/MS spectrum obtained from a spiked milk sample. The same conditions can also be used for the analysis of beef samples.
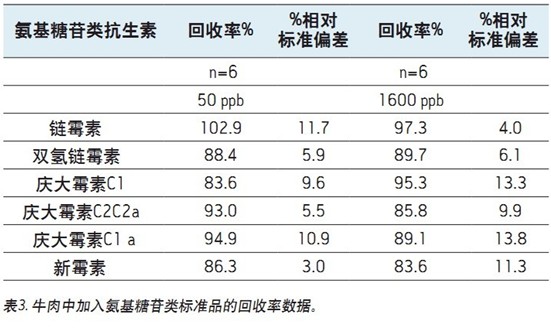
Table 2 (dairy) and Table 3 (muscle tissue) show the results obtained from the spike experiment. Neomycin (B and C) and gentamicin (C2a and C2)
It appears as an isomer and is partially isolated. The peak areas of these samples were summed for quantitative analysis.
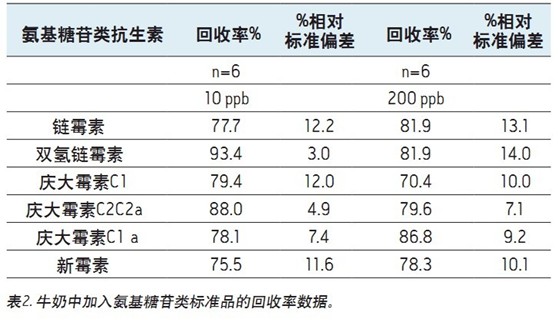
The recovery is determined by comparing the MRM peak area of ​​the standard added to the sample matrix prior to sample preparation to the peak area of ​​the standard added to the sample matrix after all sample preparation steps have been completed.
The matrix effect of meat is less than 30% and the matrix effect of dairy is less than 12%. Unlike many other antibiotic veterinary drugs used, aminoglycoside antibiotics cannot be extracted from animal tissues and related samples using organic solvents. However, these compounds can be effectively extracted from tissues using aqueous buffers. The extraction buffer includes a reagent (TCA) that promotes protein precipitation.
The Oasis HLB solid phase extraction method uses an eluent with a low organic solvent content. Compared with other methanol-type eluents (the matrix effect of using formic acid/methanol elution is greater than 50%), the purification effect can be improved. It is apparent that after elution of the aminoglycosides with an aqueous eluent, a large amount of matrix interference components can be retained on the adsorbent.
UPLC analysis was performed using ion-pair reversed-phase chromatography mode. The best balance of peak shape and sensitivity is achieved by using 20 mM heptafluorobutyric acid as an ion pair reagent. UPLC analysis using hydrophilic interaction chromatography was also considered, however, the peak shape was not as sharp as the peak shape obtained by the selected method. In addition, a disadvantage of the hydrophilic interaction chromatography mode is that the diluent for the standard and sample should be acetonitrile. The optimized solid phase extraction method provided in this application note provides for aqueous sample injection and is more suitable for reversed phase chromatography.
in conclusion
â– Excellent UPLC performance with the ACQUITY UPLC HSS PFP analytical column for ion-pair reversed-phase chromatography.
â– Heptafluorobutyric acid (HFBA) is an effective ion-pairing reagent for UPLC analysis.
â– A simple procedure for extracting aminoglycoside antibiotics from beef and milk; an operator can easily analyze 20 samples per day.
â– An optimized solid phase extraction method using Oasis HLB 96-well plates was proposed. The detection limit for milk sample analysis is 10 ppb, and the detection limit for beef samples is 50 ppb; for all analytes at this level, the analytical signal-to-noise ratio is greater than 100:1.
references
1. Screening and Confirmation for Aminoglycosides by UHPLC-MS-MS
(United States Department of Gariculture Food Safety and Inspection Service,
Office of Public Health Science).
2. Plozza T, Trenerry VC, Zeglinski P, Nguyen H, Johnstone P. The Confirmation and Quantification of Selected Aminoglycoside Residues in Animal Tissue
And Bovine Milk by Liquid Chromatography Tandem Mass Spectrometry.
International Food and Research Journal. 2011; 18(3): 1077-1084.
FDA Certified Kids Face Masks
Product Dimesnion: 5.7" x 3.7" /14.5cmx9.5cm
Mask Fabric Material: 3-ply material,100% polypropelyne
Ear Loop Material: 70% polyamide, 30% elastane
when to use: suitable for kids to use daily in a variety of settings such as home, outdoors and school
packaging: 10 pcs of mask in one sealed printed plastic bag to ensure hygiene or 50ps per box
certification/standard: GB/T 38880-2020(China children disposable mask standard) and FDA registered
about the manufacturer: Kapanou is china-based healthcare company
kids face mask,disposable kids face mask,fda approved kids face mask,fda approved face mask for kids
Ningbo Debeida Science&Technology Co.,ltd. , https://www.dbdmask.com