Hepatology: m6A RNA methylase METTL3 promotes the development of liver cancer
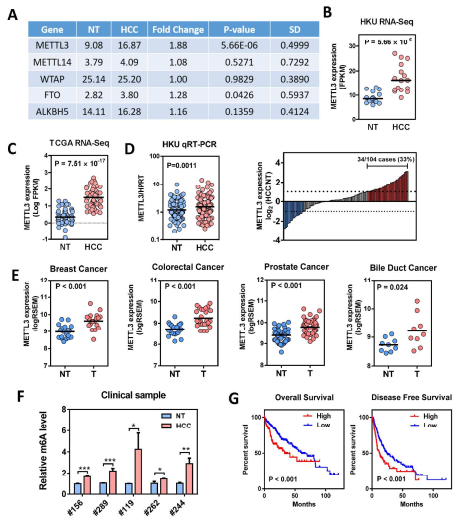
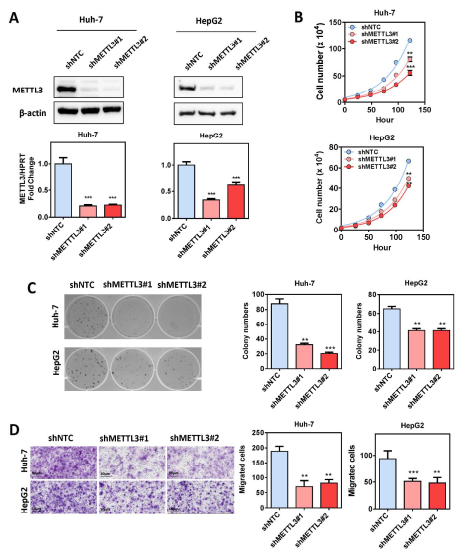
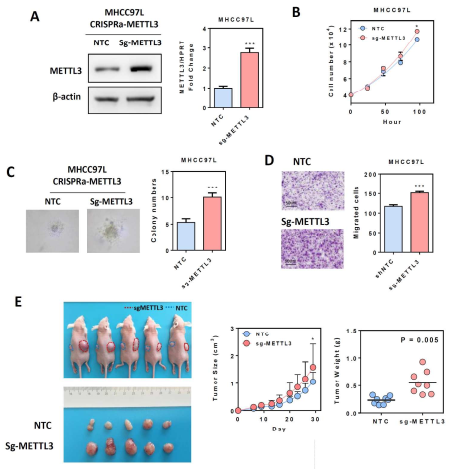
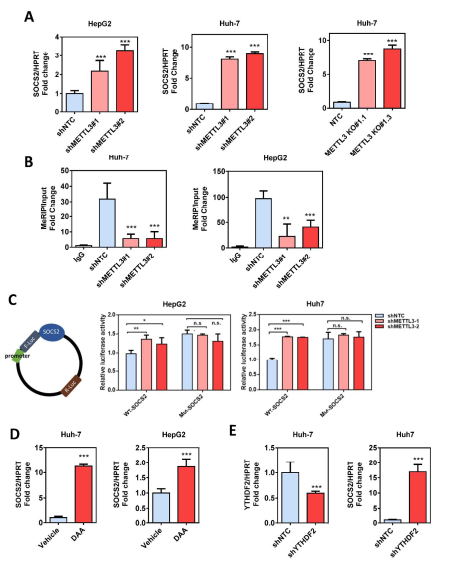
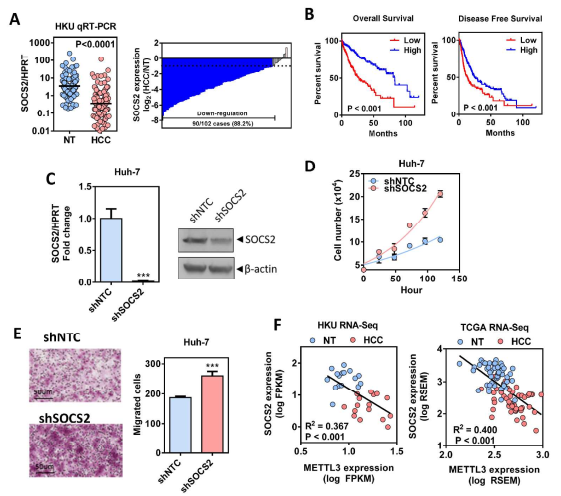
Guide In recent years, research on m6A RNA modification has become one of the most popular research directions in the field of life sciences. With the publication of CNS articles, the molecular mechanism of catalysis, removal and recognition of m6A RNA modification is becoming more and more clear. What seems to be more concerned is the correlation between m6A RNA modification and major diseases such as tumors. Epigenetic changes have greatly contributed to the development of human cancer, a recent article in Hepatology entitled " RNA N6-methyladenosine methyltransferase METTL3 promotes liver cancer progression  Through YTHDF2 dependent post-transcriptional silencing of SOCS2† The article reports the role of m6A modification in liver cancer. In the present study, the authors found that the m6A methylase METTL3 was significantly up-regulated in human liver cancer and other malignancies by transcriptome sequencing, and its overexpression predicted a poor clinical outcome. In vitro experiments show that METTL3 can promote the proliferation, invasion and colony formation of liver cancer cells. In vivo experiments have also shown that METTL3 can promote the ability of liver cancer to form tumors and lung invasion. Next, by comparing transcriptome sequencing, m6A sequencing and MeRIP-qPCR results, the authors found that SOCS2 can be used as a METTL3-mediated target gene for m6A modification. Knocking out METTL3 disrupts the level of RNA methylation in SOCS2 and upregulates its mRNA expression levels. The authors also found that m6A-mediated degradation of SOCS2 mRNA is dependent on the m6A reading protein YTHDF2. This study provides a new dimension for the study of apparent levels of liver cancer. 1. m6A methylase METTL3 is up-regulated in liver cancer and predicts poor clinical outcome By performing RNAseq on 16 pairs of HBV-associated liver cancer and corresponding non-cancer tissues, the authors found that METTL3, a core component of m6A methyltransferase, was significantly up-regulated, which is consistent with data from 50 pairs of liver cancer patients in the TCGA database. Next, the authors performed qPCR verification on 104 liver cancer and normal liver tissues, and found that the results were consistent with the sequencing results, METTL3 was significantly up-regulated, and its m6A level was also significantly high expression. In addition, high expression of METTL3 was significantly associated with poor prognosis, suggesting that up-regulation of METTL3 in liver cancer may promote the development of liver cancer. Illustration: Expression of METTL3 in liver cancer and its relationship with viability 2.METTL3 promotes the development of liver cancer The authors stabilized METTL3 in HepG2 and Huh-7 cells stably by shMETTL3#1 and shMETTL3#2. In vitro experiments showed that knockdown of METTL3 inhibited the proliferation, colony formation and invasion of hepatoma cells. In addition, knockdown of METTL3 was found to inhibit tumorigenic ability and growth rate by subcutaneous tumor experiments. In addition, the author also overexpresses the endogenous METTL3 of MHCC97L cells through the CRISPR/dCas9-VP64/MS2-p65-HSF1 gene activation system, and found that METTL3 overexpression can promote the proliferation and invasion of liver cancer. In summary, METTL3 can promote the development of liver cancer. Illustration: knocking down METTL3 can inhibit the proliferation and invasion of liver cancer cells Illustration: Knockdown/except METTL3 inhibits proliferation and invasion of liver cancer cells Illustration: Overexpression of METTL3 can promote the development of liver cancer 3. RNA-seq and m6A-seq identify SOCS2 as a target gene downstream of METTL3- mediated m6A modification  Next, by comparing transcriptome sequencing, m6A sequencing and MeRIP-qPCR results, the authors found that SOCS2 can be used as a METTL3-mediated downstream target gene for m6A modification.  Schematic: RNA-seq and m6A-seq identify SOCS2 as a target gene downstream of METTL3-mediated m6A modification 4. METTL3 by m6A reader YTHDF2 dependent pathway inhibition of mRNA stability SOCS2 The authors first confirmed by MeRIP-qPCR that the mRNA of SOCS2 is regulated by the m6A modification of METTL3. To explore how m6A modification affects SOCS2 expression, the authors constructed wild-type and mutant SOCS reporter mini genes. For the mutation reporting mini gene, A is replaced by C in the consensus sequence of m6A (such as RRACH), thereby eliminating the m6A modification. The authors found that mutations in SOCS2 significantly increased the expression of SOCS2. By comparing the fluorescence activity, it was also found that the fluorescence intensity of the wild-type SOCS2 fusion human reporter gene was significantly amplified after silencing METTL3. However, knocking down METTL3 had no effect on the mutant SOCS2 fusion reporter gene. The above results indicate that the expression level of SOCS2 is regulated by the m6A modification of METTL3. In addition, from the sequencing results, the authors found that the YTHDF2 binding site is very close to the m6A modification site. Therefore, the authors found that SOCS2 expression was significantly up-regulated by knocking down YTHDF2. All of the above results confirmed that METTL3 inhibits the mRNA stability of SOCS2 through the YTHDF2-dependent pathway of the m6A reader. Illustration: The apparent silence of SOCS2 is achieved by the METTL3-m6A-YTHDF2-dependent mechanism. 5. SOCS2 inhibit the development of liver cancer In this study, it has been found that METTL3 knockdown can increase the mRNA and protein expression of SOCS2. Therefore, the authors performed qPCR on 102 pairs of liver cancer tissues and corresponding normal liver tissues, and found that SOCS2 was significantly down-regulated in liver cancer cells, and 88.2% of them. The down multiplier is greater than 2. Through in vitro experiments, the authors found that SOCS2 can be known to develop liver cancer. Combined with the TCGA database, it was found that SOCS2 was negatively correlated with METTL3 expression. In summary, SOCS2 is a target gene downstream of METTL3 and plays an inhibitory role in liver cancer. Schematic: SOCS2 as a tumor suppressor gene, negatively correlated with METTL3 expression References : RNA N6-methyladenosine methyltransferase METTL3 promotes liver cancer progression through YTHDF2 dependent post-transcriptional silencing of SOCS2 Security Safe Box,Dual Alarm Safe Box,Security Office Safes,Security Home Safes Hebei Yingbo Safe Boxes Co.,Ltd , https://www.yingbosafebox.com


 Â
  Â
Â