Wearable Monitoring and Diagnosis Study of Intel and MJFF in Parkinson's Disease
Release date: 2014-08-21 Intel collaborated with the Michael J. Fox Foundation (MJFF), a non-profit organization, on Parkinson's disease to study the symptoms patterns of Parkinson's patients. In this multi-phase study, patients used wearables to monitor their symptoms, while researchers used a big data analytics platform to analyze the collected information. Intel said at the product launch that there is currently no monitoring to diagnose Parkinson. At present, people can only pass many doctors' examinations, and often need to wait a few years to have accurate diagnosis results. Symptoms of greatest interest to researchers include tremors, exercise, balance, slow gait, and quality of sleep. Intel explained that, under ideal conditions, participants have been wearing equipment to maximize the collection of relevant information for research. The correlation between symptoms can help researchers understand the progression of neurodegenerative diseases and also help them relate to disease and molecular changes. This is the second study between Intel and MJFF this year. The first study was to analyze the feasibility and data of the wearable devices used in the experiments. The trial involved 16 Parkinson's patients and normal volunteers. The participants wore the equipment for four days and went to the clinic twice. Intel did not specify which wearable devices were used in the lab, but the company has experience working with wearables companies. In March 2014, Intel partnered with sports tracker manufacturer Basis Science. MJFF has been working on Parkinson's disease for several years. In May 2013, the organization announced that it would use mobile phones to collect data and use machine learning algorithms to analyze data to improve Parkinson's disease research. Source: Kexun Medical Network Closed System Suction Catheter
Closed Suction System
Helping reduce cross-contamination than the traditional suction catheter.
Suction Catheter,Suction Catheter System,Suction Catheter Tube,Medical Suction Catheter Hangzhou Trifanz Medical Device Co., Ltd , https://www.cfzmeds.com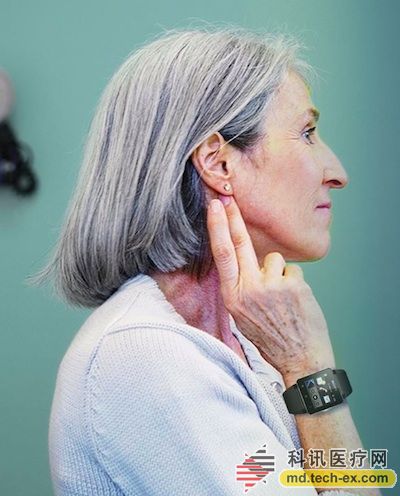
Built-in spring of vacuum control, finger-on for suction, finger-off for closing.
7-tone date indication to remind the replacement.
Sizes available from Fr5-Fr16.