Introduction to the principle and selection of drug refrigerators
Principle and selection of drug refrigerator working principle The refrigerant (R134a) is pressed into a high-pressure gas by a compressor, and then becomes a high-pressure liquid through a condenser (the refrigerant liquefies and releases heat, so that both sides of the external machine are hot during cooling), and becomes a low-pressure cryogenic liquid through the capillary. Then, it becomes a low-pressure gas through the evaporator, and the refrigerant vaporizes and absorbs heat. Therefore, the inside is cool during cooling, and then the refrigerant returns to the compressor again to repeat the above work. Cooling method (direct cooling and air cooling) [The difference between air cooling and direct cooling] [Direct cold and air cooling advantages and disadvantages] The new version of GSP "Pharmaceutical Management Quality Management Regulations" for retail pharmacy storage requirements Related legislation: [Drug refrigerator selection] Drug refrigerator application place 1. CDC, health center, hospital laboratory, blood station, pharmaceutical factory, school laboratory Microbiotic
Microbiotic, Nitazoxanide Antibiotic,Ceftiofur Sodium,Ceftiofur Sodium Sterile Powder,Imatinib Mesylate Powder,
Henrikang Biotech is specialized in Research, Analysis, Production, Application, Process and Sales. We insist in innovation and produce Microbiotic, Nitazoxanide Antibiotic,Ceftiofur Sodium,Ceftiofur Sodium Sterile Powder,Imatinib Mesylate Powder Xi'an Henrikang Biotech Co.,Ltd , https://www.henrikangbio.com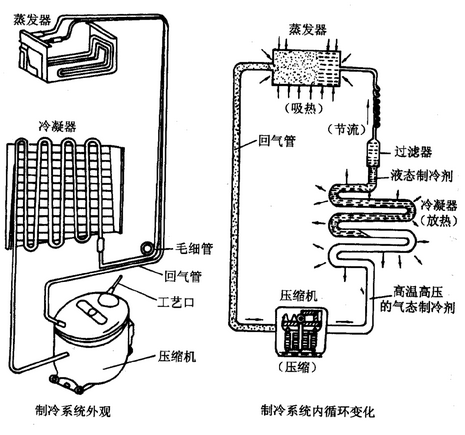
Air-cooled: A fan is installed inside, and the evaporator is placed in a special cooling area. The air is sent to the refrigerator through the fan and the air duct to cool directly: the natural convection of the air in the refrigerator is used for cooling. The air around the evaporator is exchanged heat with the evaporator, which transfers heat to the evaporator, which transfers the cooling to the air. The cold and hot air thus recirculates through natural convection to achieve the purpose of refrigeration. Since the evaporator is the inner wall of the refrigerator, the defrosting system cannot be installed, so manual defrosting is required.
Direct cooling: low noise, low power consumption, suitable for storage of fruits and vegetables, but need defrosting air cooling: fast cooling, no frosting, noise and power consumption is larger than direct cooling, suitable for storing pharmaceutical reagents 
1. Pharmacy warehouse, refrigerated and frozen medicines are implemented in accordance with GSP 151-154.
2, cool storage of drugs in accordance with the Pharmacopoeia standards.
Article 142 The enterprise shall establish relevant records such as drug procurement, acceptance, sales, display inspection, temperature and humidity monitoring, and treatment of unqualified drugs, so as to be true, complete, accurate, effective and traceable.
Article 148 The business premises shall have the following business equipment:
(2) Equipment for monitoring and regulating temperature;
(4) For the operation of refrigerated medicines, there are special refrigeration equipment;
Article 164 The display of drugs shall meet the following requirements:
(1) Display and display according to the dosage form, use and storage requirements, and set the target mark, the category label is clear and accurate;
(8) The refrigerated medicines are placed in the refrigerating equipment, the temperature is monitored and recorded according to the regulations, and the storage temperature is guaranteed to meet the requirements.
1. According to the pharmacy's own situation, customer budget and Chinese model specifications to buy
2. Suggest township pharmacies, usually open the door. In the county urban area, at least double doors.
3. According to the pharmacy area, below 60m2, open the door. 60-100m2, double door.
More than 100m2, three open doors.
4. According to the regulations of the local food and drug administration.
2. Hospital pharmacy, chain single pharmacy has passed GSP certification
high quality products. Our main products are Top quality APIs Raw Material, Best price Herbal Extract, High Quality Sarms Body
Building, Wholesale price Cosmetic Raw Material, Professional and reliable Veterinary raw Material, natural product Food Supplement,
Loss Weight Powder, research and so on.
Now we have 3 GMP standard workshop, Meanwhile, Healthcare Product factory is equipped with the researching and quality
inspection centre, with strong technology research and development strength. Blood Pressure raw powder have 3 sales departments
over 30 people to sell our products all over the world. For customer`s requirements, OEM / ODM service is available. Such as customized
bottled capsules, blister, etc.Organic Hemp Protein have excellent quality.The best-selling product is Glutathione Oral Solution.Let
customers have a consistent praise of the product is Humectants Cosmetic Raw.Henrikang adhere to the concept of [Green, Natural
and Health", sincerely hope to strengthen exchanges and cooperation with you.