Application principle and precautions of CBS denaturing gradient gel electrophoresis DGGE
Principle : Denaturing Gradient Gel Electrophresis (DGGE) is a practical method for studying DNA mutations. The essence is a kind of electrophoresis (electrophoresis definition: charged particles move under the action of an electric field toward an electrode opposite to its electrical potential, called electrophoresis (EP). The technique of using charged particles to move in different speeds in an electric field to achieve separation is called electrophoresis. technology.) In addition, DGGE can also be used in combination with PCR, which can accurately identify single base substitutions, frameshift mutations and deletion mutations of less than 10 bases. PCR amplification is usually performed first using the patient's genomic DNA or DNA from a mutant individual, and then analyzed by DGGE. The human gene screened by DGGE was the globin site, and many sites were screened later, such as growth factor VIII gene and growth factor IX gene. And the method is suitable for large-sample screening, and is also useful for screening some rare mutants. Another effect of PCR-DGGE is to analyze the mutational composition, ie the mutational profile. It is mainly to use a mutagen to in vitro mutagenize human primitive lymphocyte strains to generate a large number of HPRT-mutants, and then perform PCR amplification and analyze with DGGE. The quantitative PCR-DGGE method can also be used to determine the copy number of a particular DNA sequence in the specimen. The principle is to co-amplify the internal reference of the known copy number with the target gene in the specimen, and then DGGE separates the amplification products of both, and the copy number of the target gene in the specimen can be determined according to the ratio. Mix. Autoclave for 20-30 minutes. Store at room temperature. Degas for 10-15 minutes. Filter through a 0.45 u filter. Store at 4 °C in a brown bottle for approximately 1 month. Degas for 10-15 minutes. Filter through a 0.45 u filter. Store at 4 °C in a brown bottle for approximately 1 month. A 100% denaturant solution requires re-dissolving after storage. Place the bottle in a warm bath and stir for faster Results. 5. 10% Ammonium Persulfate Store at –20 °C for about a week. Store at room temperature. Reagent 2. Glue: Second, running electrophoresis: DNA samples move in denatured colloids Precautions: Use the following configuration map to understand the functions of the following configurations: CBS DGGE configuration corresponding diagram: Shanghai Yuansheng national exclusive agent products, more electrophoresis models please refer to: http:// A medium-sized cob of corn provides more than 10% of our daily dietary fibre requirements.
Fibre is fermented by bacteria in the colon. Promising studies are underway to determine the health-promoting effects of fibre fermentation breakdown products, for example, short-chain fatty acids, which may help to maintain a healthy gut.
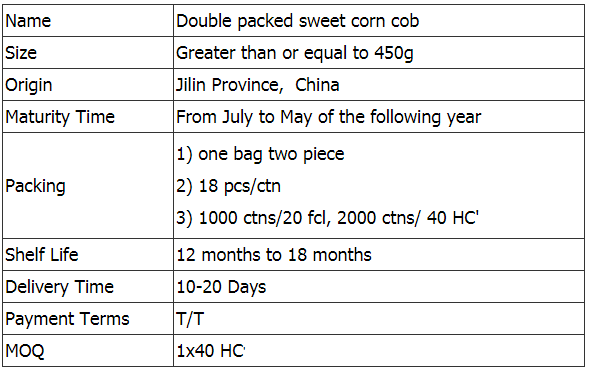
Yellow Sweet Corn,Double Packed Sweet Corn,Double Packed Sweet Corn Cob,Double Packed Yellow Sweet Corn Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorns.com
DGGE mainly uses gradient gelatin to separate DNA fragments. When electrophoresis begins, the mobility of DNA in the gel is only related to the size of the molecule. Once the DNA moves to a certain point and reaches the denatured concentration position of the DNA, the DNA double strands begin to separate, thereby reducing the migration rate of DNA. Since different DNA fragments have different base compositions, the denaturation conditions are different, and different bands are formed on the gel, so that the DNA mutation type and the wild type can be distinguished, and the gene mutation analysis can be further performed.

step:
First, the modified plastic: gradient rubber glue generator to make denatured glue.
1. Preparation solution (reagent preparation):
1. 40% Acrylamide/Bis (37.5:1)
2. 50× TAE Buffer Reagent Amount Acrylamide 38.93g Bis-acrylamide 1.07g dH2O To 100.0 ml
Reagent Amount Final Concentration Tris base 242.0g 2 M Acetic acid, glacial 57.1 ml 1 M 0.5 M EDTA, pH 8.0 100.0 ml 50 mM dH2O To 1,000.0 ml Â
3. 0% Denaturing Solution  6% Gel 8% Gel 10% Gel 12% Gel 40% Acrylamide/Bis 15ml 20ml 25 ml 30 ml 50×TAE Buffer 2 ml 2 ml 2 ml 2 ml dH2O 83 ml 78 ml 73 ml 68 ml Total volume 100 ml 100 ml 100 ml 100 ml
100% Denaturing Solution  6% Gel 8% Gel 10% Gel 12% Gel 40% Acrylamide/Bis 15 ml 20 ml 25 ml 30 ml 50×TAE Buffer 2 ml 2 ml 2 ml 2 ml Formamide(deionized) 40 ml 40 ml 40 ml 40 ml Urea 42 g 42 g 42 g 42 g dH2O To 100 ml To 100 ml To 100 ml To 100 ml
4. For denaturing solutions less than 100%, use the volumes for acrylamide, TAE and water described above in the 100% Denaturing Solution. Use the amounts indicated below for urea and formamide Denaturing Solution 10% 20% 30% 40% 50% 60% 70% 80% 90% Formamide (ml) 4 8 12 16 20 twenty four 28 32 36 Urea(g) 4.2 8.4 12.6 16.8 twenty one 25.2 29.4 33.6 37.8 Reagent Amount Ammonium persulfate 0.1 g dH2O 1.0 ml
6. 2× Gel Loading Dye Reagent Amount Final Concentration 2% Bromophenol blue 0.25 ml 0.05% 2% Xylene cyanol 0.25 ml 0.05% 100% Glycerol 7.0 ml 70% dH2O 2.5 ml  Total volume 10.0 ml Â
7. 1× TAE Running Buffer Reagent Amount 50× TAE buffer 140 ml dH2O 6,860 ml Total volume 7,000 ml Denaturing gel Gel
8. Volume Reagent Volume 0%UF Calculated according to the required denaturation range, a total of 12.5 ml ddH2O 3.9ml 100% UF 50× TAE 100 uL APS 25 uL 40% Acrylamide 1 mL TEMED 15 uL APS 10 uL Prepare a high concentration, a low concentration, each volume of 12.5 mL, the operation should be rapid, the necessity of pumping and ice bath during preparation is not great TEMED 8 uL Current use
Take 12.5 mL of two denaturants at high and low concentrations, add them to two tube troughs of two gradient generators, add high concentration to the right tube trough, add low concentration to the left tube trough, and then add cross-linking agent (add APS first, Add TEMED). The solution is poured into the two glass plates in the gel box by a peristaltic pump, and the solution is inserted into the comb after the solution is filled with the glass plate, and the solution is solidified into a gel after being left for a while.
1. Add enough buffer to the tank and preheat to the specified temperature.
2. Put the solidified gel cartridge into the tank, start to spot, set up the electrophoresis apparatus, and run the electrophoresis.
1. When disposing reagents, be sure to use deionized water. The various containers used for making the membranes should be washed with deionized water to prevent chloride ion contamination.
2. Gluing is the key to the experiment. When filling the glass plate, use a peristaltic pump to inject the gel solution into the glass plate at a constant rate.
3. Immediately after filling the gel, clean the syringe to prevent acrylamide from solidifying and blocking the tube.
4. The DGGE electrophoresis buffer should exceed the “RUN†scale and do not exceed the “Maximam†scale.
5. When spotting, use a small syringe and put it into the bottom of the spotted hole.
6. After each use of the instrument, clean it in time and clean the glassware such as glass plate culture dish.
1. The tank is used to add a buffer to provide an electrophoretic environment.
2. The electrophoresis device is used to power the electrophoresis and set the time.
3. The gel cartridge is used to fix the glass plate and one piece is placed in the tank.
4. The safety cover can be automatically powered off by the user.
5. Heater/Agitator/Buffer Circulator is used to heat control temperature and maintain temperature uniformity.
6. The glass plate, the edge banding strip, the edge banding strip, the spring clip, the peristaltic pump and the gradient generator form a potting device.
7. The comb is inserted into the upper layer of the filled glue to be solidified and then pulled out to produce a sample mouth.
8. The siphon pump can easily drain the buffer in the tank.
9. The thermometer is used to calibrate the temperature.
Standard: 2-Place DGGE System (DGGEK-2001-220)
1. 1 reinforced buffer tank/lower reservoir with platinum electrodes and attached anode power lead
2. EPS-300-II Power Supply (see page 26) Electrophoresis System
3. 2 single gel cassette/upper reservoir with platinum electrodes
4. Glass safety cover with electrical interlock
5. Heater/Stirrer/Buffer Cycler: Heating element and thermostat maintain excellent temperature stability (±0.2°) and Minimally thermal stratification during DGGE.Buffer Cycler eliminates need of an external peristaltic pump to recycle buffer Heater/Agitator/Buffer Cycle Device
6. 4 sets of spacers (2 sets for vertical DGGE and 2 sets with injection ports for perpendicular DGGE gel casting)
7. 4 combs (2 each 1-well combs and 2 each 16-rectangular well combs)
8. Buffer siphon pump
9. 2 sets of glass plates
10. MPP-100 Mini-peristaltic pump for gradient gel formation
11. GM-40 Gradient maker, 20mls per side Gradient Glue Generator
12. 4 Gel Wrap® gaskets
13. White spring clamps
14. Thermometer thermometer

There are two types of dietary fibre - soluble and insoluble - and sweet corn contains both.
According to the American Heart Association, dietary fibre as part of an overall healthy diet can help lower blood cholesterol levels and may reduce the risk of heart disease. It is insoluble fibre that binds to cholesterol, preventing it from being absorbed into the bloodstream.
Insoluble fibre is responsible for promoting regularity and helping to prevent constipation by speeding up the passage of food and waste through the intestines and absorbing water to keep stools soft. Insoluble fibre has been shown to reduce the risk of haemorrhoids.
Fibre-containing foods such as sweetcorn also help to provide a sense of satiety and may therefore help to suppress appetite and aid weight management.
Dietary fibre has also been linked to a reduced risk of type 2 diabetes. A diet rich in fibre helps patients manage their disease.