Hydrogen peroxide vapor (HPV) bioavailability
Hydrogen peroxide vapor (HPV) bioavailability ©Bioquell UK Ltd (2011). All rights reserved Technical Report HPV Biovalidity 069/11 Hydrogen peroxide vapor (HPV) sterilization is characterized by no residue (the decomposition products are only water and oxygen) and requires only a lower ambient temperature, and the steam generation phase increases the utility of the sterilization process. Hydrogen peroxide vapor (HPV) has been sterilized for many organisms and different grades of organisms. Experiments have shown that hydrogen peroxide vapor (HPV) has a broad-spectrum bactericidal effect on microorganisms including bacteria, viruses and fungi. . The effectiveness of hydrogen peroxide vapor (HPV) for the killing of bacterial spores that are ubiquitous on environmental surfaces has been proven to be iterative, and is therefore the first in the Spaulding classification. The organisms listed in the document are classified broadly (eg bacteria, viruses and fungi) and are classified according to their microbial characteristics. Experiments have shown that the use of hydrogen peroxide vapor (HPV) to disinfect microorganisms can achieve a 6-log kill rate. table of Contents 1. Tested organisms and references/sources 1.1 Bacterial and bacterial spores 1.2 virus 1.3 phage 1.4 Fungi 1.5 Nematodes and protozoa 1.6 other 2. References / Sources 1. Tested organisms and references / sources 1.1 Bacterial and bacterial spores 1.6 other i Evidence suggests that HPV can inactivate protein-prone particles (38). i Important studies have shown that HPV plays a crucial role in the molecular biology of fragmented DNA (39). 2. References / Source 1. Rogers, JV, CL Sabourin, YW Choi, WR Richter, DC Rudnicki, KB Riggs, ML Taylor, and J. Ch a ng. 2005. Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus Spores on indoor surfaces using a hydrogen peroxide gas generator. J. Appl.Microbiol. 99:739-748. 2. Bacillus anthracis (Anthrax) deactivation investigation. Defence Science and Technology Laboratory (Dstl), Porton Down UK. 2002. 3. Kokubo, M., T. Inoue, and J. Akers. 1998. Resistance of common environmental spores Of the genus Bacillus to vapor hydrogen peroxide. PDA.J.Pharm.Sci.Technol. 52:228 - 231. 4. McDonnell, G., G. Grignol, and K. Antloga. 2002. Vapour-phase hydrogen peroxide decontamination of Food contact surfaces. Dairy.Food.Environ.Sanitat. 22:868-873. 5. Spaulding, EH 1972. Chemical disinfection and antisepsis in the hospital. J. Hosp. Res. 9:5 - 31. 6. Rickloff, JR and reliski, PA 1989. Resistance of various micro-organisms to vaporized hydrogen peroxide in Prototype tabletop sterilizer. 89 th General Meeting of the American Society for Microbiology (ASM). Orleans. 7. Cabinet bio-decontamination trial. March 1995. Health Protection Agency (previously Centre for Applied Microbiology and Research), Porton Down UK. 1995. 8. US Environmental Protection Agency (EPA) registered steril a nt. EPA registration number 72372-1 - 86703. 2009. 9. Johnston, MD, S. Lawson, and JA Otter. 2005. Evaluation of hydrogen peroxide vapour as a method for the Decontamination of surfaces contaminated with Clostridium botulinum spores. J. Microbiol. Methods. 60:403 - 411. 10. Boyce, JM, NL Havill, JA Otter, LC McDonald, NM Adams, T. Cooper, A. Thompson, L. Wiggs, G. Killgore, A. Tauman, and J. Noble - Wang. 2008. Impact of hydrogen peroxide Vapor room decont a mination on Clostridium dif f icile environmental contamination and transmission in a Healthcare setting. Infect.Control.Hosp.Epidemiol. 29:723-729. 11. Otter, JA and GL French. 2009. Survival of nosocomial bacteria and spores on surfaces and inactivation By hydrogen peroxide vapor. J.Clin.Microbiol. 47:205 - 207. 12. Case study f rom a room bio-decontamination at Imperial College School of Medicine. 2003. Contact Bioquell for further details. 13. Hall, L., JA Otter, J. Chewins, and NL Wengenack. 2007. Use of hydrogen peroxide vapor for deactivation of Mycobacterium tuberculosis in a biological safety cabinet and a room. J. Clin. Microbiol. 45:810 - 815. 14. French, GL, JA Otter, KP Shannon, NM Adams, D. Watling, a nd MJ Parks. 2004. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J.Hosp.Infect. 57:31-37. 15. Otter, JA, M. Cummins, F. Ahmad, van Tonder C., and YJ Drabu. 2007. Assessing the biological ef f icacy and Rate of recontamin a tion following hydrogen peroxide vapour decontamination. J.Hosp.Infect. 67:182-188. 16. Jeanes, A., G. Rao, M. Osman, and P. Merrick. 2005. Eradication of persistent environmental MRSA. J.Hosp.Infect. 61:85-86. 17. Dryden, M., R. Parnaby, S. Dailly, T. Lewis, K. Davis - Blues, JA Otter, and AM Kearns. 2008. Hydrogen peroxide vapour decontamination in the control of a polyclonal meticillin-resistant Staphylococcus Aureus outbreak on a surgical ward. J.Hosp.Infect. 68:190-192. 18. Determination of the effectiveness of VPHP against methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis and Bacillus stearothermophilus. Health Protection Agency (previously Centre for Applied Microbiology and Research), Porton Down UK. 2001. 19. Assessment of the ef f icacy of vapour phase hydrogen peroxide as a room disinfectant. Health Protection Agency (previously Centre for Applied Microbiology and Research), Porton Down UK. 2003. 20. Otter, JA, S. Yezli, MA Shouten, AR van Zanten, G. Houmes - Zielman, and M. Nohlmans-Paulssen. 2010. Hydrogen peroxide vapor (HPV) decontamin a tion of an intensive care unit to remove Environmental reservoirs of multidrug-resist a nt Gram - negative rods during an outbreak. Am.J.Infect.Control, accepted. 21. Bates, CJ and R. Pearse. 2005. Use of hydrogen peroxide vapour for environmental control during a Serratia Outbreak in a neonatal intensive care unit. J.Hosp.Infect. 61:364-366. 22. Rogers, JV, WR Richter, MQ Shaw, a nd YW Choi. 2008. Vapour - phase hydrogen peroxide inactivates Yersinia pestis dried on polymers, steel, and glass surfaces. Lett.Appl.Microbiol. 47:279-285. 23. Otter, JA, J. Chewins, D. Windsor, and H. Windsor. 2008. Microbiological contamination In cell culture: a potential role for hydrogen peroxide vapour (HPV)? Cell.Biol.Int. 32:326-327. 24. Rickloff, JR 1990. Use of Vapourized Hydrogen Peroxide for the Bio-decontamination of Enclosed Areas. Interphex USA Con f erence. New York. 25. Heckert, RA, M. Best, LT Jordan, GC Dulac, DL Eddington, and WG Sterritt. 1997. E f ficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl.Environ.Microbiol. 63:3916-3918. 26. Adenovirus deactivation trials. Conducted in commercial confidence. 2003. Contact Bioquell for Further details. 27. Viral deactivation trials. Conducted in commercial confidence. 2002. Contact Bioquell For further details. 28. McDonnell, G., Belete, B., Fritz, C., and Hartling, J. 2001. Room decontamination with vapour hydrogen Peroxide VHP for environmental control of parvovirus. American Association for Laboratory Animal Science (AALAS), Annual meeting. Baltimore, MD. 29. Rudnick, SN, JJ McDevitt, MW First, and JD Spengler. 2009. Inactivating Influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. Am.J.Infect.Control. 37:813-819. 30. Investigation into the efficacy of hydrogen peroxide vapour in the bio-deactivation of Dengue virus. 2003. Conducted in commercial confidence. Contact Bioquell for f urther details. 31. Otter, JA, M. Barnicoat, J. Down, D. Smyth, S. Yezli, and A. Jeanes. 2010. Hydrogen peroxide vapor (HPV) Decontamination of an intensive care unit room used to treat a patient with Lassa fever. J.Hosp.Infect.in press. 32. Otter, JA and A. Budde-Niekiel. 2009. Hydrogen peroxide vapor: a novel method for the environmental Control of lactococcal bacteriophages. J.Food.Prot. 72:412 - 414. 33. Pottage, T., C. Richardson, S. Parks, JT Walker, and AM Bennett. 2010. Evaluation of hydrogen peroxide Stable disinfection systems to decontaminate viruses. J.Hosp.Infect. 74:55 - 61. 34. Information supplied with kind permission of Eli Lilly and Company, Indianapolis, Indiana. 1996. 35. Hall, L., JA Otter, J. Chewins, and NL Wengenack. 2008. Deactivation of the dimorphic f ungi Histoplasma capsulatum, Blastomyces dermatitidis and Coccidioides immitis using hydrogen peroxide vapor. Med.Mycol. 46:189-191. 36. Gustin, EJ, McDonnell, GE, Mullen, G., and Gordon, BE 2002. The efficacy of vapour phase hydrogen Peroxide against nematode infestation: the Caenorhabditis ele g ans model. American Association for Laboratory Animal Science (AALAS), Annual meeting. San Antonio, Texas, USA 37. Krause, J. and Riedesel, H. 2002. Elimination of pinworm eggs from caging equipment with vapourised Hydrogen peroxide. Report from the Max-Planck - Institute for experimental medicine. Association for Laboratory Animal Science (AALAS) national meeting. San Antonio, Texas, USA 38. Fichet, G., K. Antloga, E. Comoy, JP Deslys, a nd G. McDonnell. 2007. Prion inactivation using a new gaseous Hydrogen peroxide sterilisation process. J.Hosp.Infect. 67:278 - 286. 39. DNA bleeding studies. Conducted in commercial confidence. 2008. Contact Bioquell For further details. Bioquell's hydrogen peroxide vapor (HPV) has been widely used in various countries around the world as a biological disinfectant due to its broad-spectrum sterilization and rapid inactivation of microorganisms. Frozen Seafood Mix,Mixed Seafood Bags,Frozen Seafood Mix Bags,Frozen Seafood Cocktail Zhoushan Haiwang Seafood Co., Ltd. , https://www.haiwangseafoods.com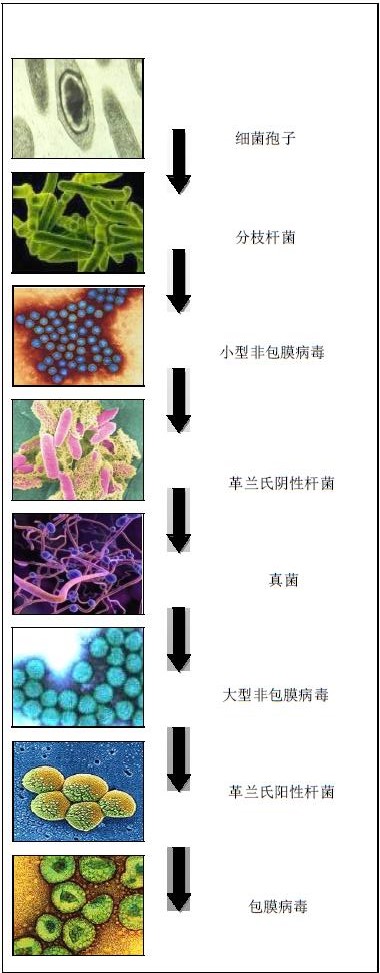
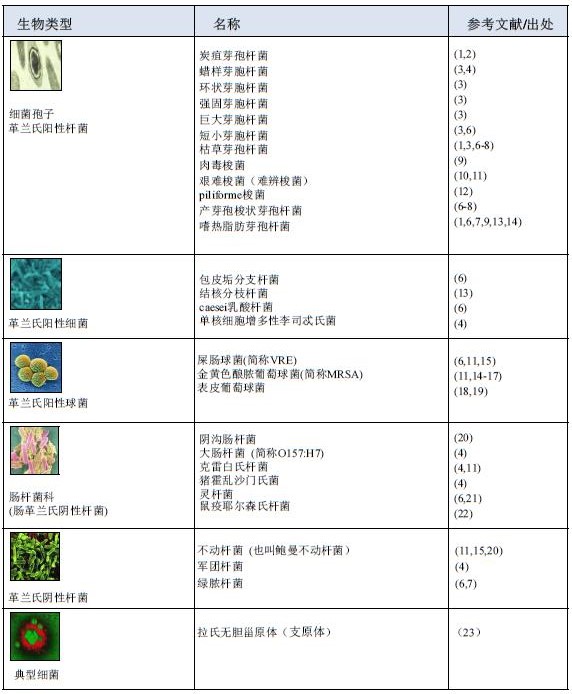 1.2 virus
1.2 virus 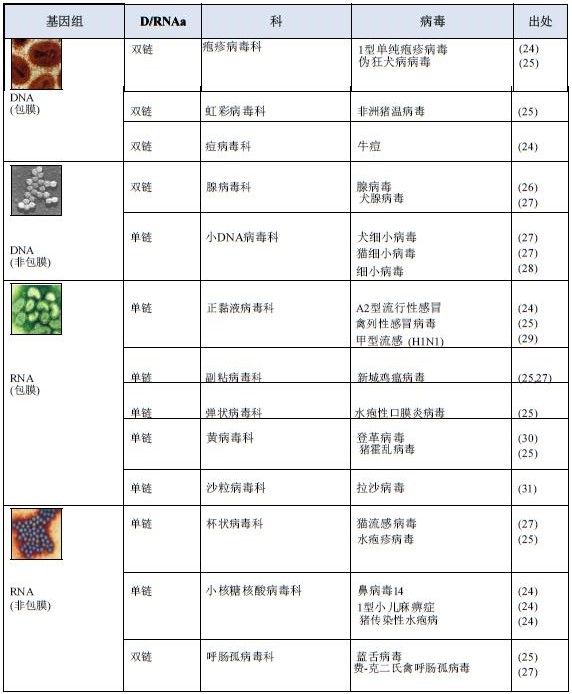 1.3 phage
1.3 phage  1.4 Fungi
1.4 Fungi 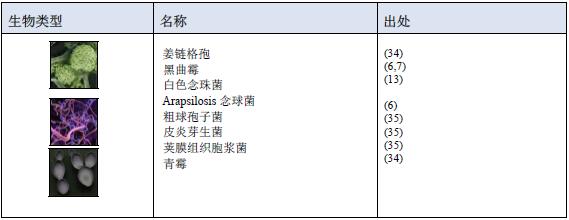 1.5 Nematodes and protozoa
1.5 Nematodes and protozoa 
