Molecular Devices detects cell viability or toxicity on cell imaging systems
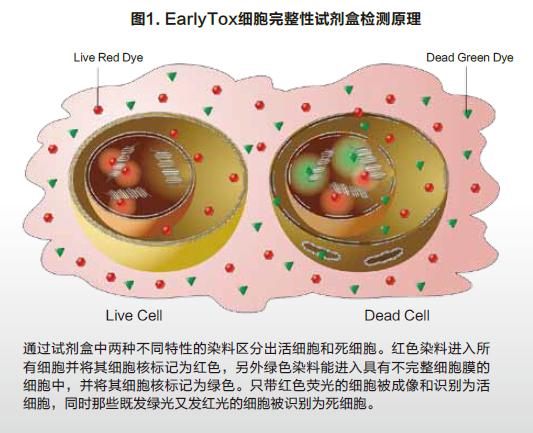
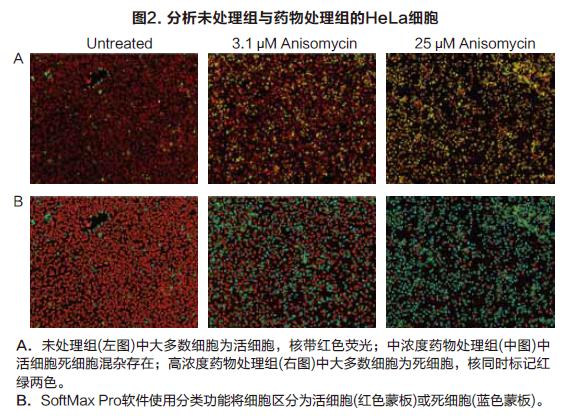
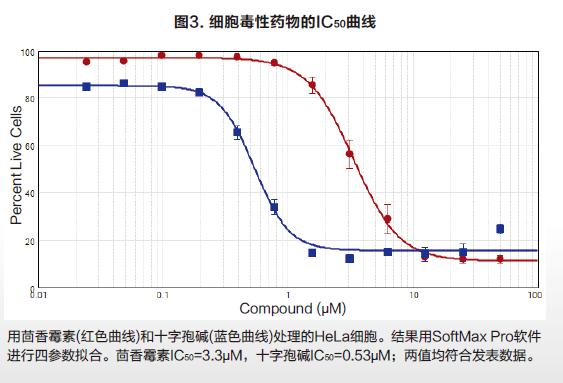
Molecular Devices detects cell viability or toxicity on cell imaging systems The EarlyToxTM Cell Integrity Kit consists of a series of optimized reagents that easily identify living and dead cells. It can be used to examine the effects of different treatments on cell viability and to assess the cytotoxicity of different mechanisms, including apoptosis and necrosis. The kit is designed to work in a variety of cell types, both in adherence and suspension. Simple process flow and excellent reagent performance make it suitable for high-throughput scanning. Two DNA-binding fluorescent dyes are used in the kit, one is a cell membrane-penetrating living cell red dye, and the other is a dead cell green dye that is impenetrable to the cell membrane. The red dye enters the nucleus of all cells, and the cell membrane is intact and has no effect on it, and can be excited to produce a bright red fluorescent signal. Green dyes can only enter dead cells and produce bright green fluorescence, and the cell membranes of such cells are incomplete (Figure 1). Therefore, fluorescent signals captured by living cells and dead cells through the imaging system are easily distinguished and recognized. The SpectraMaxTM MiniMaxTM 300 Cell Imaging System and SoftMax® Pro software provide a complete suite of hardware software tools for imaging and data analysis for EarlyTox Cell Integrity Kit applications. Using the instrument's dual-channel fluorescence imaging capabilities and software's single-cell analysis capabilities, a 96-well plate cell activity can be analyzed in 5 minutes. Characteristics Strong signal, shortened exposure time, faster imaging Suitable for a wide range of cell types, widely used Photographing and analysis are completed, streamlined method HeLa cells were seeded at a density of 5000 cells/well into a black-walled 384-well plate, cultured for 24 hours to adhere and grow, and then cytotoxic drugs were added to the wells. At 24 hours of drug treatment, the cell activity was measured by adding a dye label according to the kit instructions. The method of operation should be as uniform as possible to reduce cell loss and inaccuracy of results due to removal of media, rinsing steps. An optional masking agent can be used to reduce the background value of the medium or test drug. result High-throughput cell imaging of 384 wells was performed using the green (541 nm emission) and red (713 nm emission) channels of the SpectraMax MiniMax 300 cell imaging system (Fig. 2A). The image imaged by the red channel sets the object size and fluorescence threshold, and the software's target recognition function automatically identifies all nuclei in each image. The software's classification function will automatically identify and count live and dead cells by distinguishing only red-colored nuclei and simultaneously labeled red-green nuclei (Fig. 2B). Finally, a series of detailed data can be output, such as the percentage of living cells in each well as a percentage of total cells. The SoftMax Pro software performs a 4-parameter fit to obtain an IC50 curve for drug concentration and cell viability (Figure 3). in conclusion At the same time, the SpectraMax MiniMax Cell Imaging System and the EarlyTox Cell Integrity Kit can be used to quickly and accurately detect the number of dead cells and living cells by fluorescence imaging. The cytotoxicity caused by various cellular mechanisms can be identified and quantified. SoftMax Pro software can distinguish between different staining modes of living cells and dead cells, and can automatically curve the data without complicated data import and export, saving data analysis processing time. Neomycin Sulphate Tulle Dressing Neomycin Sulphate Tulle Dressing,Neomycin Sulfate Gauze Dressing,Sulfate Tulle Dressing,Neomycin Sulfate Tulle Dressing Roosin Medical Co.,Ltd , https://www.roosinmedical.com